What does evidence-based medicine mean actually? It is defined as conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients (1). We have witnessed dramatic reduction in the mortality rate of diseases beginning from the half of the 20th century. This improvement in the life expectancy can be attributed not only to the advances in the healthcare but also to the well designed and conducted large randomized clinical trials (RCT’s), in which effective therapies have been tested and implemented to the practice only after establishing clinical efficacy. We should not forget what has been taught as one of the first things in medical schools: “Primum non nocere”, first do not harm.
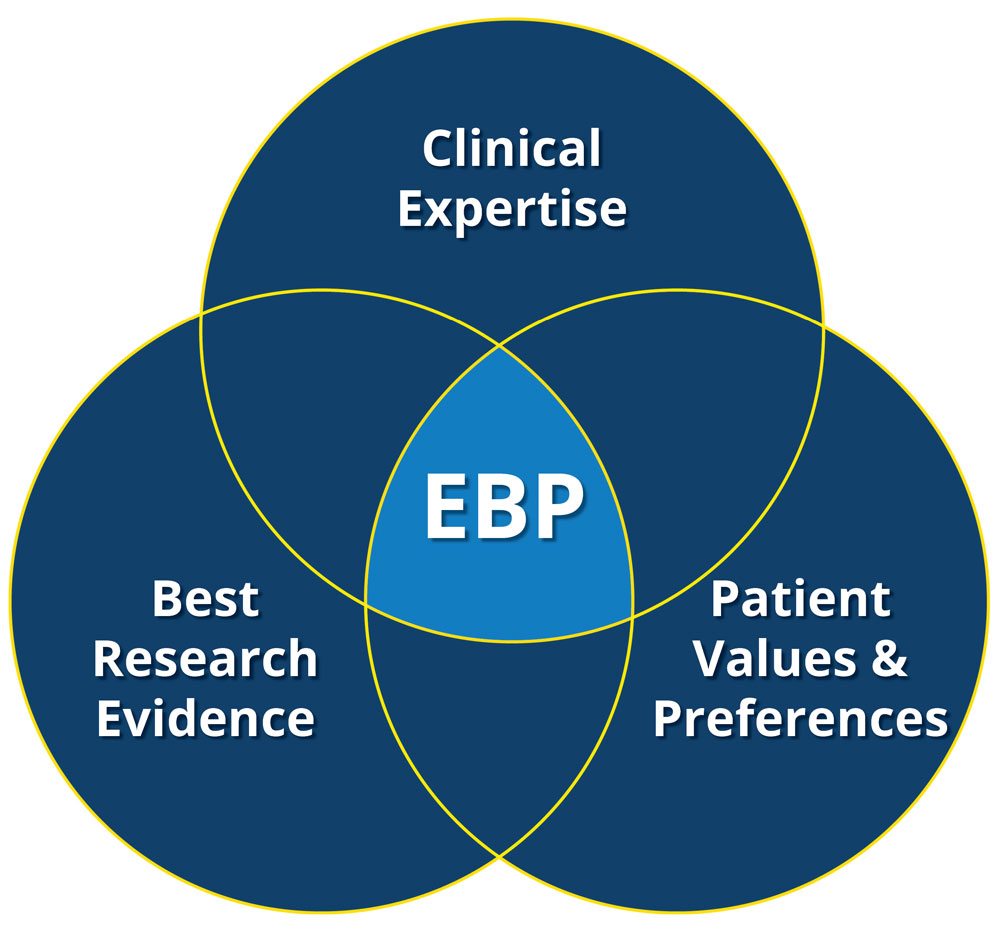
RCT’s are considered as the most reliable method for generating clinical evidence. Why is that? Well, the most important reason is a properly designed and executed RCT eliminates majority of confounders, which can potentially interfere with results. We have plenty number of bitter experiences that should remind us how confounders and bias in personal experiences, established routines, probable mechanistic treatment benefits or even observational study results contradicted with the findings from RCT’s. One clear example was the recommendation of the hormone replacement therapy (HRT) to the post-menopausal women based on results from observational therapies. Subsequent RCT results indicated that in fact the HRT increased coronary heart diseases and invasive breast cancer (2), which lead to abandon recommending the HRT in this population.
You may ask what is to do with COVID-19? To answer that let’s go back to the very first weeks of the pandemic (I know you do not want but just for a second) and think about what medical advices and opinions you came across in the social media and television. Here are some examples; - “Turkish people will not be affected from the virus as Chinese were, because the former have inherent genetic protection”, - “Vitamin C and Zinc are the only medications that you need to take for the protection from the virus” and - “Wearing mask will not help in reducing the spread of the virus”. Although many of these were not taken seriously in the society we can’t say the same thing for many other advices.
The most striking example of why evidence based medicine is important was the prophylactic use of the anti-malarial drug, hydroxychloroquine (HCQ). It got more dramatic after Donald Trump announced (1) that he was taking the drug for prophylaxis as his physician justified by stating “… the potential benefit from treatment outweighed the relative risk”. But… Where was the evidence? Ok… a tiny observational study (36!!! patients) from France found that 20 patients who were treated with HCQ had lower viral load compared to 16 patients who did not take drug (4). Let’s give a brief pause here for a second and do some critical appraisal. Six patients in the treatment group were lost to follow up. What were the reasons for lost to follow up? Three were transferred to the intensive care unit (ICU), 1 died, 1 left the hospital and 1 stopped the treatment. What were the reasons for ICU transfer in three patients? Could it be due to Torsades de Pointed secondary to drug-induced QT interval prolongation? We do not know but might be.

It was only several weeks that people had to wait for seeing the evidence and unfortunately these were not in the same direction with the tiny study or Donald Trump’s and his physician’s way of thinking. First came findings from several observational studies suggesting no benefit of HCQ alone or in combination with azithromycin in the treatment for COVID-19 (5,6). We knew that, as a golden rule of evidence based medicine, findings from observational studies should not imply a practice change; instead they should be treated as hypothesis generating and tested in a randomized clinical trial. Then, results from the randomized, double-blind clinical trial which included individuals with moderate-high risk exposure to COVID-19 indicated that HCQ did not prevent confirmed COVID-19 infection within 4 days after exposure (7).
Another wound that evidence based medicine took during COVID-19 was the extreme fluctuation in the attitudes of journals with high impact factor towards submitted manuscripts during COVID-19. Would someone believe that the New England Journal of Medicine could publish a study, which included only 61 patients with no randomization, no transparency in inclusion/exclusion criteria and no control group, no explanation for the exclusion of 7 patients after being included and no clear pre-defined primary endpoints (yes, I have more no’s but let’s stop here)? Unfortunately, compassionate use of remdisivir study, which was sponsored by pharmaceutical giant Gilead, was published in the journal (8).
I am well aware that these are hard times and authorities should act faster to prevent incredibly rising number of death numbers. However, one thing that we can’t make concession about is to be transparent about the truth. HCQ could have been a reasonable treatment of choice based on its mechanism of action but we had no evidence neither for its efficacy nor potential harm. In the presence of uncertainty, policies should be changed by evidence not by beliefs.
Coming up next: RECOVERY trial
REFERENCES
- 1- Sackett, David L., et al. "Evidence based medicine: what it is and what it isn't." (1996): 71-72.
- 2- Rossouw, Jacques E., et al. "Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial." Jama 288.3 (2002): 321-333.
- 3- https://www.cnbc.com/2020/05/18/trump-says-he-takes-hydroxychloroquine-to-prevent-coronavirus-infection.html
- 4- Gautret, Philippe, et al. "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial." International journal of antimicrobial agents (2020): 105949.
- 5- Rosenberg, Eli S., et al. "Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state." Jama (2020).
- 6- Geleris, Joshua, et al. "Observational study of hydroxychloroquine in hospitalized patients with Covid-19." New England Journal of Medicine (2020).
- 7- Boulware, David R., et al. "A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19." New England Journal of Medicine (2020).
- 8- Grein, Jonathan, et al. "Compassionate use of remdesivir for patients with severe Covid-19." New England Journal of Medicine 382.24 (2020): 2327-2336.